At Nutreebio, we've been manufacturing custom gummy supplements for global clients for years—from the United States and the UK to Southeast Asian markets like the Philippines and Thailand. We've helped hundreds of brands launch compliant, well-designed gummy products in highly regulated markets.
One of the most overlooked but crucial steps in supplement development is labeling. A poorly designed or non-compliant label can delay customs clearance, trigger product recalls, or even damage brand trust. That’s why we’re writing this guide—to help you understand what goes into a legally compliant and consumer-friendly gummy vitamin label. Whether you're providing your own artwork or asking our team to design it, this article gives you a checklist of what must appear on your label—and why it matters.
Here’s a quick summary of the key sections that should appear on your supplement label:
| No. | Label Module | Key Information |
|---|---|---|
| 1 | 🧾 Product Name | E.g., “Vitamin C Gummies”, “Multivitamin Gummies” |
| 2 | 🔢 Net Quantity | E.g., “60 Gummies”, must be visible on front panel |
| 3 | 📊 Supplement Facts | Serving size, servings/container, actives, %DV |
| 4 | 🔬 Ingredients List | In descending order; include function for additives |
| 5 | ⚠️ Allergen Statement | E.g., “Contains: Soy”, “May contain traces of milk” |
| 6 | 🚫 Warnings & Directions | Daily use, safety warning, target group (e.g. adults) |
| 7 | 🏭 Manufacturer Info | Name, address; use “Manufactured for” if needed |
| 8 | 🏷️ Country of Origin | E.g., “Made in China”, “Product of USA” |
| 9 | 📅 Expiration Date | Usually on bottle bottom or outer box |
| 10 | 🔍 Lot / Batch Number | Important for traceability and inspections |
| 11 | ✅ Certifications | Only if authorized (GMP, Halal, Kosher, etc.) |
| 12 | 🧪 Structure/Function Claims | E.g., “Supports immune health*”; include FDA disclaimer |
Next, I will explain in detail one by one based on some actual cases of our nutreebio.
1. Product Name
The product name is the first thing consumers notice on a gummy supplement label — and it must be clear, honest, and descriptive. It should directly reflect the nature of the product, such as:
“Vitamin C Gummies”
“Creatine Gummies”
“Multivitamin Gummies for Adults”

The name must not be misleading or imply any disease treatment or cure. For example, names like “Diabetes Relief Gummies” or “Anti-Cancer Gummies” may violate supplement labeling regulations in many countries.
When formulating your product name, make sure it:
Clearly states the primary nutrient or function.
Reflects the product format (e.g., “Gummies”).
Aligns with your target market’s expectations.
Keep it concise, compliant, and easy to understand.
Oh! There is another one, which is eye-catching!😄
2. Net Quantity
The Net Quantity indicates the total amount of product in the package and is a required element on the front panel of your gummy supplement label.
It should be expressed in either:
✅ Units (e.g., number of gummies)
Example: 60 Gummies
✅ Weight (e.g., grams or ounces)
Example: 150g or 5.3 oz
This value must appear on the principal display panel and should be easy to spot at first glance.
Why is this important? Because Net Quantity helps consumers understand how long the product will last and whether the price reflects good value. It's worth noting that Always match the Net Quantity with the total number of servings and serving size declared in your Supplement Facts panel.

For example, our creatine gummies show:
"Serving Size: 5 Gummies"
"Servings Per Container: 12"
Then the product of the values of these two parts needs to match "60 gummies".
3. Supplement Facts
The Supplement Facts panel is the core compliance section of your gummy vitamin label. It tells consumers exactly what nutrients they’re getting — and how much — in each serving.
This section must follow strict formatting and content rules depending on your target market. I will take the standards of the US FDA and the EU EFSA as an example. The following is a breakdown of what must be included:
3.1 Serving Size & Servings Per Container
Clearly state:
- Serving Size: The number of gummies per serving (e.g., 2 Gummies)
- Servings Per Container: How many servings the full bottle contains (e.g., 30)
These two values should match the Net Quantity on the front of the package. In the previous paragraph, I introduced this content using creatine gummies as an example, I’ll skip the repetition here to avoid redundancy.
3.2 Amount Per Serving (Active Ingredients)
List each active ingredient (In our Hangover Gummies, there are nutrients such as Vitamin B1 (Thiamine), Vitamin B6 (Pyridoxine), Vitamin B12 (cobalamin), Vitamin B2 (Riboflavin), etc.) along with:
✅ The exact quantity per serving (e.g., Vitamin B1 – 3.6mg)
✅ The % Daily Value (%DV or %NRV), if applicable
✅ If no %DV is established for a particular ingredient, use a symbol like: † or a footnote: “Daily Value not established.”

For example, our Hangover Gummies have a daily intake percentage of "%REI/RNI". Both REI and RNI mean recommended nutritional intake. However, REI is often used in Commonwealth countries, the Philippines, and WHO documents, while RNI is sometimes used on labels in Asian countries such as China and Malaysia.
“Vitamin Vitamin B1(Thiamine) 3.6mg – 300% RNI”
Description: Each serving contains 3.6mg of vitamin B1, which is 300% of the recommended daily intake (RNI) for men aged 19-29.
Ingredients such as Dihydromyricetin and S-Acetyl Glutathione are followed by **, indicating that there is no official recommended intake reference value for the ingredient.
Common ingredients include:
- Amino acids (such as L-Glutamine, L-Tyrosine)
- Plant extracts (such as Ashwagandha, Ginseng, Green Tea Extract)
- Functional compounds (such as Creatine, CoQ10, Collagen)
- Special nutrients (such as Hyaluronic Acid, Alpha-GPC)
Although these ingredients have clear functions, there is no "daily recommended intake" numerical basis in the traditional nutrition framework, so it must be written:
" Creatine Hydrochloride 4g – "
" % REI/RNI Not Established "
3.3 Units & Formats
Use proper nutrient units, such as:
mg (milligrams)
µg (micrograms)
IU (International Units), only for specific vitamins like D & E (FDA only)
Be consistent and ensure all units are scientifically accurate.
3.4 Formatting Tips
Use bold lines to separate sections (e.g., between macronutrients and vitamins)
Group similar items (e.g., list all B vitamins together)
Align all amounts and %DV figures for easy reading
3.5 Why It Matters
Your Supplement Facts panel is the first place regulators, retailers, and consumers look to evaluate the product’s quality and compliance. Mistakes in dosage, unit type, or formatting can lead to:
Customs delays
Label rejection
Consumer complaints
Amazon listing takedowns
✅ Tip for Brands: Always verify the %DV requirements for your target country — for example, the US (FDA), UK (FSA), and EU (EFSA) all have different reference intake values.
4. Ingredients List
The Ingredients List is a required part of your gummy label and should appear below or beside the Supplement Facts panel. While the Supplement Facts shows only active nutrients, the Ingredients List includes everything used in the formula — both actives and excipients.
4.1 What to Include
List all ingredients in descending order by weight, including:
Active ingredients (e.g., vitamins, minerals, botanicals)
Excipients (e.g., gelling agents, sweeteners, flavors, acids, oils, colorings)
4.2 Ingredient Naming Rules
Use common names that consumers can recognize.
e.g., "Vitamin C" instead of "L-ascorbic acid"
For additives, specify function in parentheses:
e.g., "Sweetener (Steviol Glycoside)"
e.g., "Coloring (Curcumin)"
⚠️ Notes:
Allergens should not be hidden here — list them in a separate Allergen Statement. I will explain the content of allergens in the next chapter. Currently, many international big-name gummy manufacturers directly use "Other Ingredients" to indicate excipients in the Supplement Facts box or below it, without a separate Ingredients List module. This practice is a mainstream industry practice in the US market and is also one of the formats accepted by the FDA. But in stricter countries such as the EU (under EFSA guidance) or Canada, then I recommend that supplement product labels must have both Supplement Facts (or Nutrition Information) and a separate Ingredients List.

5. Allergen Statement
An allergen statement is essential for consumer safety and regulatory compliance. It must clearly indicate if your gummy product contains or may contain any of the top food allergens.
5.1 Common Allergens to Declare
- Soy
- Milk
- Eggs
- Tree nuts
- Peanuts
- Wheat
- Fish
- Shellfish
- Sesame (required in the US since 2023)
5.2 Required Phrases (Examples)
Use clear, standardized statements:
“Contains: Soy and Tree Nuts”
“May contain traces of Milk and Peanuts”
“Produced in a facility that also processes Wheat and Eggs”
These statements are especially important in private-label and export products, where different regulatory bodies (e.g., US FDA, EU, UK FSA) may have strict allergen labeling rules.
5.3 Gummy-Specific Considerations
In gummy manufacturing, allergens may come from:
Gelatin (if derived from fish or pork – important for Halal/Kosher labeling)
Flavorings or coatings (may contain dairy or soy derivatives)
Cross-contamination from shared equipment
5.4 Where to Place It
Place the allergen statement immediately after the Ingredients List, using bold or highlighted text for visibility. This is a key requirement in markets like the US, EU, and UK.
6. Warnings & Directions for Use
This section helps protect consumer safety and reduces regulatory risks. It includes how the product should be used, who it is intended for, and any cautionary statements.
✅ What to Include:
6.1 Recommended Use (Dosage & Frequency):
Example: “Suggested use: Take 2 gummies before consuming alcoholicbeverages or as directed by a healthcare professional. For bestresults,chew thoroughly before swallowing. Do not exceed therecommended dosage.”
Ensure the dosage reflects the quantities listed in the Supplement Facts panel.

6.2 Intended User Group:
Clearly state the age or user group:
“For adults only”
“Not intended for children under 12”
“Consult your doctor if pregnant, nursing, or taking medication”
Here’s what our Hangover Gummies label says: "CAUTION: Pregnant or nursing mothers, children under the ageof 18, and individuals with a known medical condition shouldconsult a physician before using this or any dietary supplement.Please drink legally,responsibly and never drink and drive."
- Mandatory Warnings:
Our label: "KEEP OUT OF REACH OF CHILDREN.DO NOT USE IF SAFETY SEAL IS DAMAGED OR MISSING.STORE IN A COOL DRY PLACE"
There are several other forms of expression:
“Do not exceed the recommended daily intake.”
“Keep out of reach of children.”
For sugar alcohols: “Excessive consumption may produce laxative effects.”
These statements are required in markets like the US and EU and help reduce legal risks.
7. Manufacturer / Distributor Information
This part ensures product traceability and is required by law in most countries.
This section is relatively simple and requires the company name, address, and contact information.
The company name needs to be the full legal name. The address must contain at least the city, state/province, and country/region. In some regions (such as the European Union), a full postal address is required. Contact information is mainly an email, website, or customer service phone number.
8. Country of Origin
The Country of Origin indicates where the gummy vitamins are manufactured or processed. It’s a legal requirement in many countries and provides transparency for consumers.
Example: “Made in China”, “Product of USA”, or “Manufactured in Germany”
Must be accurate and not misleading.
Often placed near the manufacturer’s address or at the bottom of the back label.
Some markets (e.g., EU, US) enforce strict labeling rules for imported supplements, so accurate origin disclosure is critical for customs clearance and compliance.
9. Expiration / Best Before Date
The Expiration Date (or “Best Before” date) tells consumers how long the product remains safe and effective if stored properly.
Example: “Best before: 2026/12/31”
Usually printed on the bottom of the bottle, back of the pouch, or in the corner of the label.
Should be paired with storage instructions if relevant (e.g., “Store in a cool, dry place”).
This helps assure product quality, safety, and shelf-life—key concerns in regulated supplement markets.
In the image below, our sample shows EXP: 05-2027, meaning the product is guaranteed stable and effective until the end of May 2027.
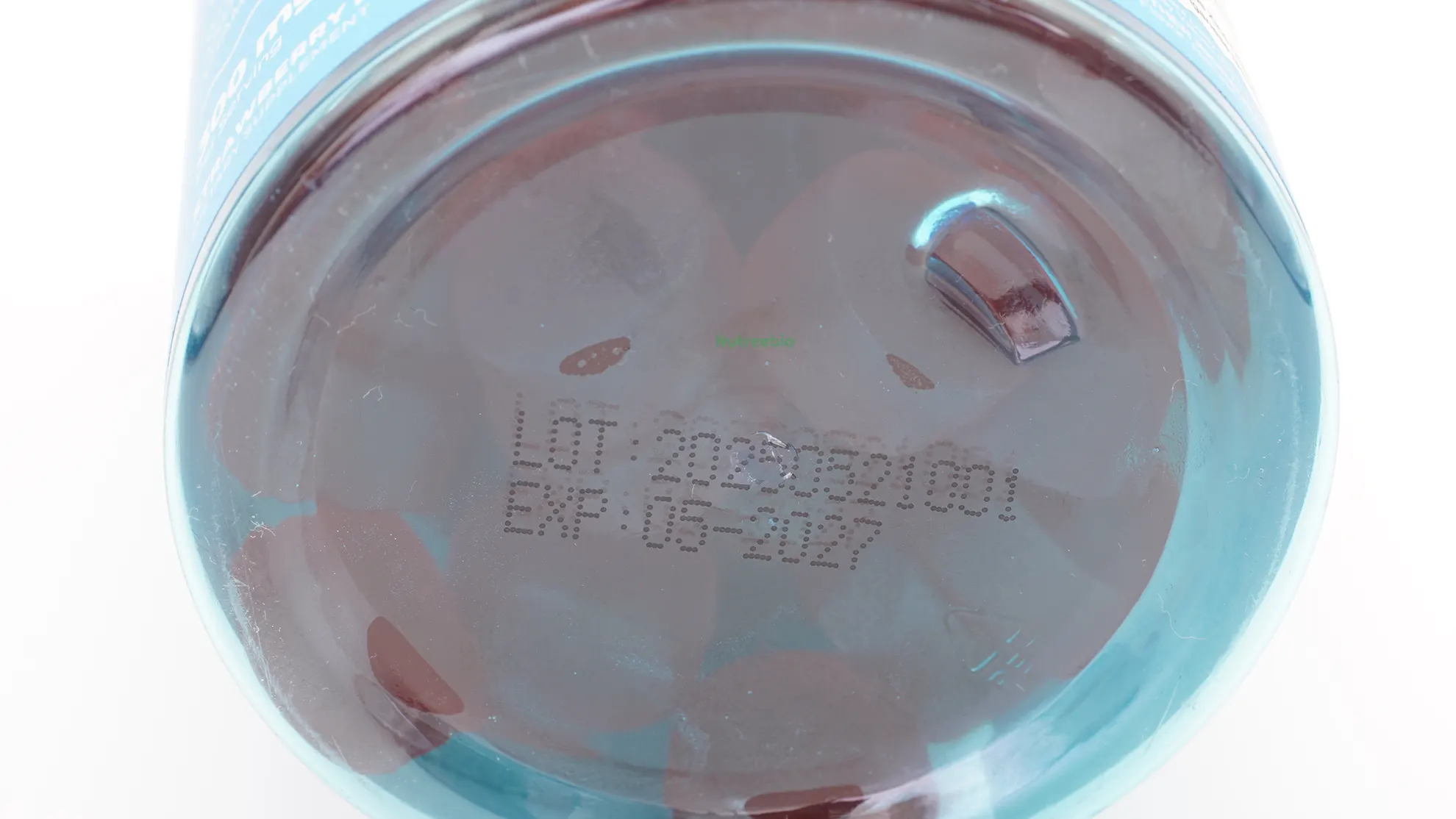
10. Lot / Batch Number
The Lot Number (also called Batch Number) is a unique code assigned to a specific production run. It enables full traceability in case of quality audits or product recalls.
Example: “Lot: 202406A”
Usually printed or laser-coded near the expiration date.
Required for GMP-compliant and export products, especially to the US, EU, and Australia.
It’s a critical quality control tool and a required element in nearly all supplement labeling guidelines.
In our gummy label, the Lot number is 20250521001, meaning this batch was produced on May 21, 2025, and is the first run of the day.
11. Certification Logos
Certification logos enhance consumer trust and signal adherence to quality, safety, and ethical standards. These logos must only be used if your product or facility has officially obtained the certification.
Common examples include:
GMP (Good Manufacturing Practice) – Regulatory requirement in many countries, especially for exports to the US and EU.
Halal / Kosher – Important for religious or cultural dietary preferences.
Vegan / Vegetarian – Indicates the product contains no animal-derived ingredients (note: not all gummies qualify).
Non-GMO – Appeals to clean-label-conscious consumers.
Gluten-Free / Allergen-Free – Important for individuals with food sensitivities.

On our labels, we display 5 certification logos: 100% NATURAL, VEGAN, NON GMO, HALAL, ALLERGEN FREE.
⚠️ Do not add certification logos unless your company has proper documentation. Mislabeling may lead to customs seizure or market penalties.
12. Structure/Function Claims
Structure/Function Claims are statements that describe how a nutrient or ingredient affects the structure or function of the body—but without claiming to treat or cure diseases.

Examples:
✅ Liver Support
✅ Pain & Inflammation Relief
✅ Electrolyte Replenishment
✅ Amino Acids For Recovery
In the US, these claims are allowed under FDA regulations only if accompanied by this mandatory disclaimer:
“These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease.”
📌 Tip: These claims should be truthful, not misleading, and ideally supported by credible scientific references. Some countries, like those in the EU, only allow pre-approved claims with specific nutrient thresholds.
🏁 Conclusion
Gummy vitamin labeling isn’t just about design—it’s about compliance, clarity, and consumer trust. With over a decade of experience exporting gummies to the US, UK, EU, Southeast Asia, and beyond, NutreeBio understands the regulatory nuances across markets.
Whether you're creating your own label or working with our in-house design team, this guide equips you with everything you need to ensure your product’s packaging is:
Clear
Compliant
Credible
Need help verifying your supplement label for your target market? Let our regulatory experts assist you in building a brand that not only looks great but also passes inspection anywhere in the world.


