The sugar substitute revolution never rests. Over the past century, artificial sweeteners have undergone six rounds of updates, gradually being replaced by natural sweeteners. The recent "Aspartame" controversy has accelerated the pace at which artificial sweeteners are being replaced.
As a long-standing star ingredient among natural sweeteners, Steviol glycosides' demand continues to grow. According to data from Innova Market Insights, in the past decade (2011-2021), the global launch of new products containing Steviol glycosides has grown at a compound annual rate of 21.9%. Most of these new products are concentrated in North America, Asia, and Western Europe. Beverages remain the primary category for the introduction of Steviol glycosides-containing products. Additionally, sports nutrition, dairy, snacks, and gummy products have seen significant growth. New categories such as desserts, ice creams, and baked goods are also gradually getting involved.
Steviol glycosides are gaining attention in the food and beverage industry due to their natural properties and almost zero-calorie value. However, their bitter and adverse aftertaste remains a significant obstacle to their application. Therefore, improving taste and physicochemical properties (such as purity and solubility) is the research focus of the next-generation Steviol glycosides.
1. "The Third-Generation Natural Zero-Calorie Healthy Sugar Source"
Steviol glycosides are a class of sweetening substances extracted from Stevia rebaudiana, commonly referred to as the "third-generation natural zero-calorie (zero-calorie) healthy sugar source" worldwide. Stevia rebaudiana is a perennial shrub native to Paraguay, Brazil, and Argentina. For centuries, local people have used Stevia rebaudiana leaves for medicinal purposes and to sweeten beverages, such as Mate tea. Botanist Moises Santiago Bertoni introduced Stevia rebaudiana to the rest of the world in 1887. To date, commercial cultivation of Stevia rebaudiana is carried out in countries such as Argentina, Brazil, Colombia, Paraguay, China, Japan, Malaysia, South Korea, Vietnam, Israel, Australia, Kenya, the United States, and Europe. China is the world's largest exporter of Stevia rebaudiana, accounting for 80% of global production, with the United States being a major destination for Chinese Stevia rebaudiana exports.
In 1931, French chemists Bridel and Lavielle isolated Steviol glycosides from Stevia rebaudiana. Globally, over 40 Steviol glycosides have been identified. Typically, the top three Steviol glycosides in Stevia rebaudiana in terms of content are Stevioside (4-13% w/w total dry weight), Rebaudioside A (2-4% w/w total dry weight), and Rebaudioside C (1-2% w/w total dry weight). Rebaudioside D and Rebaudioside M are found in very low quantities in Stevia rebaudiana (approximately 0.4-0.5% w/w total dry weight).
The bitter and adverse aftertaste of Stevioside and Reb A restrict their application, and Reb D and Reb M are gaining attention as the main categories of next-generation Steviol glycosides.

2. Next-Generation Steviol Glycosides
2.1 Reb D or Reb M
Due to the low natural content of Reb D and Reb M in Stevia rebaudiana, it is challenging to achieve commercial production. Scientists are continually trying various methods to increase the yield of Reb D and Reb M. Currently, there are three main production technologies, including breeding, bioconversion, and fermentation. In bioconversion and fermentation, the enzymes or microorganisms used are removed from the final product, leaving purified Steviol glycosides.
A. Breeding Method
The breeding method involves cultivating Stevia rebaudiana varieties with high Reb M, Reb D, or other specific glycoside content. It is a more natural approach but requires a significant trial and error period. Spectrum Chemicals' Starleaf Stevia can produce more Reb D and Reb M, approximately 20% higher than conventional methods. Recently, the Haotian Pharmaceutical Industry developed the SoPure™ Galaxy, a 100% plant-based natural Steviol glycoside series designed for dairy applications. Haotian has been improving Stevia rebaudiana varieties for over a decade, and the main cultivated varieties include those with high Reb A content, high Total Steviol Glycosides (TSG) content, high Stevioside (STV), and high Reb M and Reb D content.
B. Bioconversion Method
The bioconversion method involves using specific enzymes to convert Reb A or Stevioside extracted from Stevia rebaudiana into the target Steviol glycosides, such as Reb M, Reb D, or other special glycosides. The enzymes come from genetically modified microorganisms, mainly non-toxic and non-pathogenic strains of yeast and Escherichia coli. These genetically modified strains can produce glucosyltransferase and sucrase.
In late 2022, Spectrum Chemicals' bioconverted Steviol glycosides (Reb D, Reb M, and Reb AM) series received approval in the European Union. In May of this year, Sweegen received full authorization for the use of bioconverted Reb M in the UK. Recently, Icon Foods launched Stevia Sweet RM95, with a concentration of Reb M not less than 95%. Stevia Sweet RM95 is manufactured in the United States and produced through the bioconversion of Stevia rebaudiana cultivated in Peru.
C. Fermentation Method
Fermentation production involves using yeast (usually genetically modified microorganisms) to ferment sugar into target Steviol glycosides, such as Reb M and Reb D, or other unique glycosides. Fermented Steviol glycosides have a refreshing and sugar-like taste, helping food and beverages maintain a consistent taste while reducing sugar. Unlike Steviol glycosides sourced from plant extracts, fermented Steviol glycosides primarily use glucose and sucrose as raw materials, ensuring stable production and supply. The cost is also lower than Steviol glycosides extracted from Stevia rebaudiana. Although the initial cost is higher, the cost advantage of fermentation production becomes apparent when establishing large-scale production. Additionally, fermentation production is scalable, and additional production capacity can be added by simply adding fermentation tanks.
In 2018, Cargill and DSM established a joint venture called Avansya to produce Reb M through fermentation. The main raw material for fermentation is corn glucose, and the yeast used is genetically engineered baker's yeast. Amyris, a California-based company, uses genetically modified yeast strains to convert sucrose into Reb M.
2.2 Glucosylated Steviol Glycosides
Sugar glycosylation is a method that selectively introduces glucose units into Steviol glycoside molecules using enzymes, usually cyclodextrin glucosyltransferase and α-amylase. This creates glucosylated Steviol glycosides, also known as enzyme-modified Steviol glycosides. The enzymes mainly come from non-toxic and non-pathogenic strains of thermophilic Bacillus, licheniformis Bacillus, and hay Bacillus. Compared to Steviol glycosides, glucosylated Steviol glycosides have advantages such as reducing the natural bitterness of Steviol glycosides and improving solubility.
Japan and South Korea were among the first countries to use glucosylated Steviol glycosides. The Japanese Food Additive Standard and the Korean Pharmacopoeia include quality standards and testing methods for glucosylated Steviol glycosides. In 2011, the FDA first announced that enzymatically modified Steviol glycosides had obtained GRAS certification and could be used as sweeteners in various foods. Subsequently, the FDA has reported on six types of enzymatically modified Steviol glycosides that have been GRAS certified. The most recent notification from the FDA concerns two enzymatically modified Steviol glycosides from Haotian Pharmaceutical Industry: 1) SoPure Stevia™ glucosylated Steviol glycosides, GSG 80 (Total Steviol Glycosides content ≥80%, with glucosylated Steviol glycosides content ≥75% and cyclodextrin content ≤20%); 2) GSG 95 (Total Steviol Glycosides content ≥95%, with glucosylated Steviol glycosides content ≥75% and cyclodextrin content ≤5%).
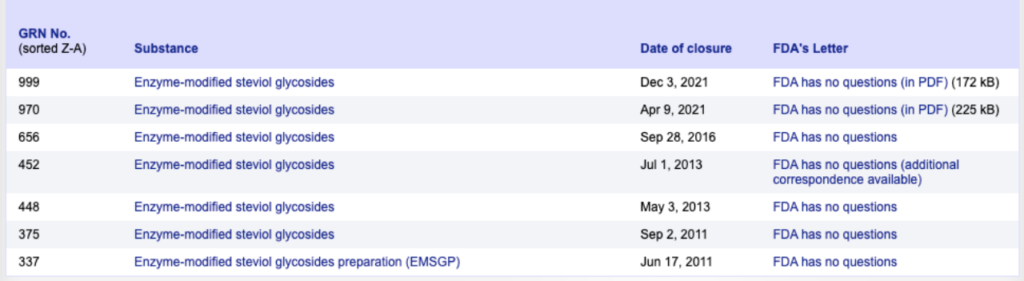
Additionally, FEMA approved enzymatically modified Steviol glycosides of various specifications for use in edible flavors in 2018. JECFA revised the framework approved in 2019 at its 91st meeting in February 2021, establishing specifications for "Enzymatically Modified Glucosylated Steviol Glycosides" among the different methods of producing Steviol glycosides. In February 2022, the European Union approved the safety assessment of glucosylated Steviol glycosides as a food additive.
In China, according to the national standard GB 2760-2014 and the National Health Commission's Notice No. 8 of 2016, glucosylated Steviol glycosides can be used as edible flavors in various foods except those listed in Table B.1 of GB 2760-2014, and can be used as needed in production.
Currently, several companies produce glucosylated Steviol glycosides, including the Tate & Lyle Group, Haotian Pharmaceutical Industry, Yingjia Heseng, BGG, Golden Health Industries, Kangmeida Biology, Aojing Biology, Qingyi Foods, and others.
2.3 The "Steviol Glycosides+" Series
A. Combination of Multiple Steviol Glycosides
Yingjia Heseng's Steviol glycosides GIAVIA Plus series combines multiple Steviol glycosides in different proportions, with a sweetness approximately 200-300 times that of sucrose. It has only about 1/125th of the calories of sucrose, is not absorbed by the body after intake, dissolves easily in water, and, compared to Reb A, has a more pronounced improvement in bitterness and aftertaste. The sweetness is pure, full, clean, with a short aftertaste, and no noticeable sourness, metallic taste, or astringency.
B. Steviol Glycosides + Erythritol
As a high-intensity sweetener, Steviol glycosides (mainly Reb A and Reb B) have a relatively slow onset of sweetness, and the sweetness increases rapidly, often resulting in a lingering aftertaste. Erythritol can alter the time course of sweetness and can eliminate some trailing effects.
Sanhe Biological's Tianlv Yuanbrand combines Steviol glycosides and erythritol using co-crystallization technology. This product combines the original functions of erythritol and Steviol glycosides. It can replace sucrose, xylitol, and other artificial sweeteners in most fields.
C. Steviol Glycosides + Alulose + Mogroside
Icon Foods combines Steviol glycosides, alulose, and mogroside to create the KetoseSweet+ brand. It is said that KetoseSweet+ has a very neutral flavor and a taste similar to sugar. It is suitable for carbonated and non-carbonated beverages, baked goods, frozen desserts, syrups, chewing gum, and candies, among other applications. It can enhance water binding and browning reactions through the Maillard reaction, among other functional improvements.
D. Steviol Glycosides + Erythritol + Mogroside + Alulose
Icon Foods also offers a mixed sweetener called Iconisweet, which combines erythritol, Steviol glycosides, mogroside, and alulose. According to Icon Foods, this mixture leverages the strengths of each sweetener while eliminating their respective weaknesses. For example, erythritol and alulose can offset the cooling sensation and endothermic properties of Steviol glycosides, reduce crystallinity, and lower the freezing point of frozen foods, all while participating in the Maillard reaction.

3. The New Era of Sugar Substitutes
In the post-pandemic era, consumers are increasingly concerned about healthy eating. According to data from iiMedia Research, 44% of consumers in China are more focused on reducing sugar and fat intake. Considering the vast number of consumers in China, the demand for healthy eating is growing faster than the global average. While consumers have a strong awareness of reducing sugar, they do not want to sacrifice sweetness. As a result, low- or zero-calorie, low- or zero-glycemic index sugar substitutes that provide sweetness without causing blood sugar spikes have become sought-after alternatives for health-conscious consumers. The demand for sugar substitutes has shifted from "adding sweetness" to "health," leading to the launch and iteration of more natural sweeteners.
However, the variety of natural sweeteners is relatively concentrated. Globally, the most commonly used natural sweeteners include Steviol glycosides, monk fruit sweeteners, sugar alcohols (such as erythritol), rare sugars (such as allulose and tagatose), and sweet proteins (such as miraculin). While there are other emerging natural sweeteners, their market share is relatively small.
Compared to other natural sweeteners, Steviol glycosides have a rich family of members, each with distinct characteristics. This continuous innovation in the Steviol glycosides family is being driven by more and more companies. As the limitations of Steviol glycosides are overcome, it may become the next erythritol and, at the same time, usher in a new era of sugar substitutes. The direction of this sugar substitute revolution remains to be seen, and we will continue to monitor its progress."
Reference:
[1]Samuel P, Ayoob KT, Magnuson BA, Wölwer-Rieck U, Jeppesen PB, Rogers PJ, Rowland I, Mathews R. Stevia Leaf to Stevia Sweetener: Exploring Its Science, Benefits, and Future Potential. J Nutr. 2018 Jul 1;148(7):1186S-1205S. doi: 10.1093/jn/nxy102. PMID: 29982648.
[2]青山资本-糖,不是那么伟大的作品|青山资本2022年中消费报告
[3]GB 1886.355-2022 《食品安全国家标准 食品添加剂 甜菊糖苷》
[4]Olsson K, Carlsen S, Semmler A, Simón E, Mikkelsen MD, Møller BL. Microbial production of next-generation stevia sweeteners. Microb Cell Fact. 2016 Dec 7;15(1):207. doi: 10.1186/s12934-016-0609-1. PMID: 27923373; PMCID: PMC5142139.


