The pursuit of health and wellness has never been more prevalent, and at the forefront of this quest are dietary supplements. These potent allies in the form of pills, powders, gummies, and softgels promise to fill nutritional gaps, boost health, and prevent disease. However, in this burgeoning market, the United States Food and Drug Administration (FDA) stands as a sentinel, ensuring that the promise of better health is backed by products that are safe and reliable.
The FDA's certification is not merely a regulatory hurdle; it is a beacon of trust for consumers. It signifies that a supplement has been subjected to a series of stringent evaluations and complies with the highest standards of quality and safety. This certification holds immense importance in the industry, serving as a critical differentiator in a sea of countless products.
For consumers, the FDA's emblem on dietary supplements is a guiding star, assuring them that what they consume is as beneficial as it claims to be. It is a symbol of assurance that the ingredients listed on the bottle are present in the right amounts and free from harmful additives. In an age where health is not just a personal goal but a lifestyle, the FDA's role in certifying dietary supplements becomes not just regulatory in nature but fundamentally essential to public health and safety.
The FDA's certification process is a testament to the agency's commitment to upholding these standards. It is a comprehensive review that scrutinizes every aspect of a supplement—from the sourcing of its ingredients to the manufacturing processes and facilities, to the accuracy and honesty of its labeling. This process ensures that the supplements reaching the consumer's hands are not only effective but also worthy of their trust and investment.
As we delve deeper into the intricacies of the FDA's certification process and its implications for the supplement industry, it becomes clear that this is not just about compliance—it's about a commitment to excellence and the wellbeing of consumers nationwide.
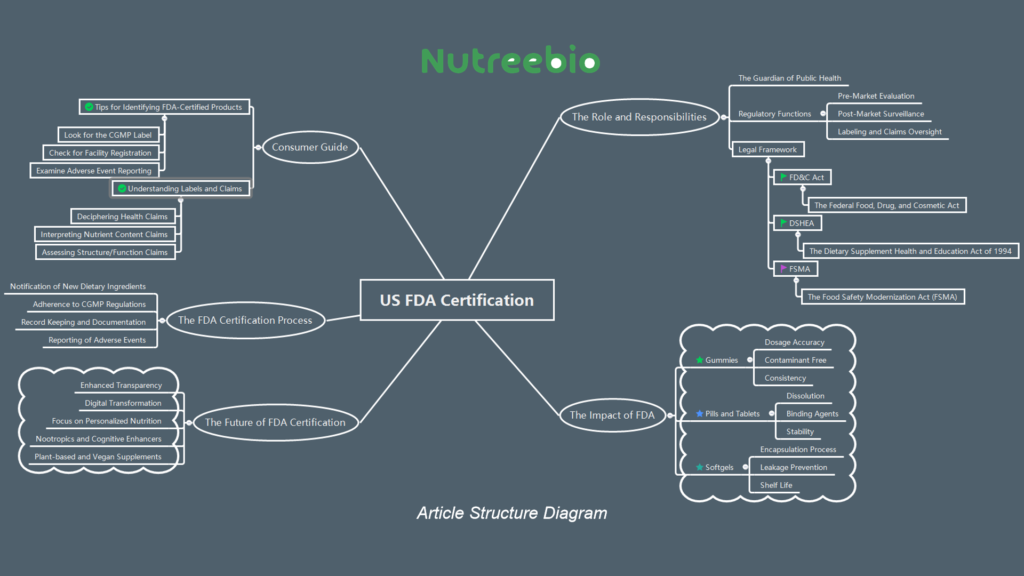
1.The Role and Responsibilities of the FDA in the Supplement Industry
1.1 The Guardian of Public Health
The FDA's role in the dietary supplement industry is multifaceted and all-encompassing. As the guardian of public health, the agency's responsibilities extend far beyond mere oversight. It is tasked with the critical mission of ensuring that every supplement sold in the United States is safe for consumption and manufactured according to the highest standards of quality.
1.2 Regulatory Functions
The FDA's regulatory functions are comprehensive:
Pre-Market Evaluation: While dietary supplements do not require pre-market approval like pharmaceuticals, the FDA is responsible for the pre-market evaluation of new dietary ingredients. Any substance that was not marketed in the United States before October 15, 1994, must be notified to the FDA with evidence supporting its safety.
Post-Market Surveillance: The FDA actively monitors the market for any adverse events reported by consumers. This surveillance is crucial in identifying and addressing potential risks associated with dietary supplements.
Labeling and Claims Oversight: The FDA meticulously reviews the labeling of supplements, ensuring that all claims made are truthful and not misleading. It also ensures that the health claims, nutrient content claims, and structure/function claims comply with FDA regulations.
1.3 Legal Framework and Regulatory Oversight
The legal framework within which the FDA operates includes several key pieces of legislation:
The Federal Food, Drug, and Cosmetic Act (FD&C Act): This act provides the FDA with authority over the safety and labeling of dietary supplements.
The Dietary Supplement Health and Education Act of 1994 (DSHEA): DSHEA specifically addresses the regulation of dietary supplements, defining what constitutes a dietary supplement and setting forth the framework for its labeling and manufacturing practices.
The Food Safety Modernization Act (FSMA): Enacted in 2011, FSMA gives the FDA new authorities to regulate the way foods are grown, harvested, and processed, including dietary supplements.
The FDA's regulatory framework is designed to protect consumers while supporting the supplement industry's ability to provide healthful products. It is a delicate balance between fostering innovation and ensuring public safety, a task that the FDA navigates with a commitment to science and consumer well-being.

2.The FDA Certification Process for Dietary Supplements
Building upon the foundation of the FDA's role, we now turn to the intricate process of FDA certification for dietary supplements, highlighting the steps and requirements, the significance of CGMP, and the regulations surrounding labeling and claims.
2.1 A Journey of Compliance and Quality
The process of FDA certification for dietary supplements is not a one-time event but a continuous journey of compliance and quality assurance. It begins with the understanding that while the FDA does not "approve" dietary supplements as it does with drugs, it does set forth a stringent set of guidelines and regulations that manufacturers must adhere to in order to market their products in the United States.
2.2 Steps and Requirements
Notification of New Dietary Ingredients: For any new dietary ingredient that was not marketed in the United States before October 15, 1994, manufacturers must notify the FDA at least 75 days before the product is introduced to the market. This notification must include evidence that the ingredient is safe for consumption.
Adherence to CGMP Regulations: Manufacturers must comply with the FDA's Current Good Manufacturing Practice (CGMP) regulations. These guidelines are in place to ensure the identity, purity, strength, and composition of dietary supplements. This includes quality control measures, proper labeling, and the prevention of adulteration.
Record Keeping and Documentation: Manufacturers are required to keep detailed records of every aspect of their production process. This includes records of each batch of product, its distribution, and any adverse event reports related to the product.
Reporting of Adverse Events: Under the Dietary Supplement and Nonprescription Drug Consumer Protection Act, manufacturers, packers, and distributors of dietary supplements must report to the FDA any serious adverse events reported to them by consumers.
2.3 The Importance of CGMP
CGMP regulations are the cornerstone of the FDA's assurance of quality in dietary supplements. They require manufacturers to evaluate their products through testing of the raw materials used, the in-process materials, and the finished products. This ensures that the dietary supplements are free from contamination and that they are labeled with the correct ingredients and dosage.
2.4 Labeling and Claims Regulations
The FDA requires that dietary supplements be labeled correctly and that any claims made about the products are accurate and not misleading. This includes:
Health Claims: Statements that describe a relationship between a dietary supplement ingredient and reducing the risk of a disease or health-related condition.
Nutrient Content Claims: Statements that describe the level of a nutrient in the product, such as "high in calcium" or "contains 100 mg of vitamin C."
Structure/Function Claims: These claims describe the role of a nutrient or dietary ingredient intended to affect the normal structure or function of the human body, for example, "supports immunity."
Manufacturers making these claims must possess evidence that the claims are truthful and not misleading, and they must include a disclaimer on the product label that the FDA has not evaluated the claim.

3.The Impact of FDA Certification on Different Forms of Supplements
The FDA's certification process casts a wide net over the diverse array of dietary supplements available in the market. Each form, from gummies to pills to softgels, is subject to stringent scrutiny to ensure that they meet the high standards set forth by the agency. This section will explore the impact of FDA certification on these various forms and its broader implications for product quality and consumer trust.
3.1 Gummies: A Sweet Path to Health
Gummy vitamins have surged in popularity, offering a palatable alternative to traditional pills. The FDA's oversight for gummies is particularly stringent due to their appeal to both adults and children. The agency ensures that:
Dosage Accuracy: Gummies must contain the amount of vitamins and minerals stated on the nutritional label within a specific margin of error.
Contaminant Free: They must be free from contaminants such as heavy metals, pesticides, and microbes to ensure safety, especially for children.
Consistency: Each batch of gummies must have a uniform mix of ingredients to prevent any variation in nutrient content from one gummy to another.

3.2 Pills and Tablets: Precision in a Small Package
Pills and tablets are the stalwarts of the supplement industry, and the FDA's certification ensures:
Dissolution: The pills must dissolve at a specific rate to ensure that the body can absorb the nutrients effectively.
Binding Agents: The use of binding agents in pills is regulated to prevent adverse health effects and ensure that they do not interfere with the supplement's efficacy.
Stability: Pills and tablets must maintain their potency and quality throughout their shelf life, as verified through stability testing.

3.3 Softgels: Encapsulating Quality
Softgels are favored for their ability to hold oils and fat-soluble vitamins. The FDA's role in certifying softgels includes:
Encapsulation Process: The process must prevent oxidation and maintain the integrity of the oil-based contents.
Leakage Prevention: Softgels must be sealed adequately to prevent leakage, which could affect dosage and stability.
Shelf Life: The FDA requires that softgels maintain their quality and potency for the duration of their shelf life, which is particularly challenging for oil-based supplements.

3.4 The Broader Impact on Quality and Consumer Trust
FDA certification has a profound impact on the overall quality of dietary supplements and the trust consumers place in them. It assures consumers that:
Quality Assurance: Products have been manufactured following rigorous standards, ensuring high quality.
Informed Decisions: Clear and accurate labeling allows consumers to make informed decisions about their health.
Safety: The risk of adverse effects is minimized, protecting consumer health.
Supporting Evidence from Research
Studies have shown that FDA oversight can lead to increased consumer confidence in dietary supplements. For instance, a research article published in the Journal of Health Communication indicates that consumers are more likely to trust and purchase supplements that are associated with FDA certification (Smith et al., 2018).
4.Facing the FDA Certification Challenge: Trials and Triumphs of Supplement Manufacturers
4.1 The Uphill Battle for Compliance
Manufacturers of dietary supplements face a rigorous path to FDA certification. The challenges include:
Understanding and Implementing CGMP: Comprehensive knowledge of the Current Good Manufacturing Practice regulations is essential. Manufacturers must implement these practices across all stages of production, a process that can be resource-intensive and requires constant vigilance.
Documentation and Record Keeping: Keeping detailed records that meet FDA standards is a critical challenge. This documentation must cover every aspect of the manufacturing process, from sourcing raw materials to post-market surveillance.
Adverse Event Reporting: Establishing a system for monitoring and reporting adverse events is not only a regulatory requirement but also a public health responsibility.
4.2 Analysis of a Success Case
One notable example is the case of a dietary supplement company that received commendation for its swift response to a potential safety issue. Upon discovering a batch of products that did not meet their internal quality standards, the company voluntarily recalled the affected products and worked closely with the FDA to ensure all compliance measures were met. This action not only prevented any harm to consumers but also reinforced the company's commitment to safety and regulatory adherence. (Source: FDA Enforcement Actions)
The journey to FDA certification is fraught with challenges, but as the success stories illustrate, it is a path that can lead to enhanced product quality, consumer confidence, and industry respect. The final sections of this article will delve into the future of FDA regulation in the dietary supplement industry and how companies can stay ahead of the curve.

5.The Future of FDA Certification: Trends and Regulatory Evolution
As the dietary supplement industry continues to evolve, so too does the landscape of FDA certification. Emerging trends and anticipated regulatory changes are shaping the future of supplement certification, with a focus on innovation, consumer safety, and transparency.
5.1 Industry Trends and Anticipated Regulatory Changes
The FDA is expected to respond to industry trends with updates to its regulatory framework, particularly in the following areas:
Enhanced Transparency: There is a growing demand for transparency in the supplement industry. The FDA is likely to implement more stringent requirements for ingredient sourcing, manufacturing processes, and supply chain management.
Digital Transformation: The FDA may leverage technology to streamline the certification process, including the use of artificial intelligence for monitoring compliance and blockchain for traceability.
Focus on Personalized Nutrition: As personalized nutrition becomes more prevalent, the FDA might develop new guidelines for genetic testing and custom-tailored supplement recommendations.
5.2 Certification Prospects for Emerging Ingredients and Products
The rise of new ingredients and novel supplement products presents both opportunities and challenges for FDA certification:
Nootropics and Cognitive Enhancers: With the increasing popularity of brain health supplements, the FDA is likely to scrutinize these products closely to ensure they are safe and their claims are substantiated.
Plant-based and Vegan Supplements: As consumer interest in plant-based diets grows, the FDA may establish specific certification processes for vegan supplements, ensuring they meet nutritional needs without animal-derived ingredients.
5.3 The Road Ahead for Supplement Certification
The future of FDA certification is poised to become more dynamic as the agency adapts to the fast-paced changes within the dietary supplement industry. Manufacturers must stay informed and prepared to meet evolving regulations to ensure their products remain compliant and competitive.
Proactive Adaptation: Companies that proactively adapt to anticipated changes in FDA regulations will be better positioned to navigate the certification process successfully.
Scientific Rigor: The FDA will likely place a greater emphasis on scientific evidence supporting the safety and efficacy of supplements, particularly for those making health-related claims.
Global Harmonization: As the dietary supplement market becomes increasingly global, the FDA may work towards harmonizing its certification standards with international regulatory bodies to streamline global commerce.
The trajectory of FDA certification is clear
Increased scrutiny, advanced technology integration, and a commitment to consumer well-being. The concluding section will offer insights into how companies can align with these trends and prepare for the future of FDA certification in the dietary supplement industry.
6. Consumer Guide: Selecting FDA-Certified Supplements
In the penultimate section of our exploration into the FDA's role in the dietary supplement industry, we turn our attention to the consumer. Understanding how to identify FDA-certified products and interpret labels and claims is crucial for making informed choices.
6.1 Tips for Identifying FDA-Certified Products
Look for the CGMP Label: Certified products often highlight their compliance with Current Good Manufacturing Practices (CGMP) on their packaging.
Check for Facility Registration: Manufacturers of FDA-certified supplements are required to register their facilities with the FDA. Consumers can search the FDA's database for this information.
Examine Adverse Event Reporting: Trustworthy companies will provide information on how to report adverse events, demonstrating their compliance with FDA regulations.
6.2 Understanding Labels and Claims
Deciphering Health Claims: FDA-certified supplements that make health claims must have supporting evidence and a disclaimer stating that the FDA has not evaluated the claim.
Interpreting Nutrient Content Claims: Claims like "high in vitamin C" are regulated by the FDA, and such statements can be trusted to accurately represent the product's content.
Assessing Structure/Function Claims: These claims do not require FDA approval, but the manufacturer must have evidence to support them and notify the FDA upon marketing the product.
7. Conclusion
The Enduring Role of the FDA in the Dietary Supplement Industry
The FDA's certification process is a cornerstone of the dietary supplement industry, ensuring that products are safe, effective, and produced with the highest quality standards. While the FDA does not "approve" supplements as it does drugs, its regulations and oversight are vital for consumer protection and industry credibility.
Final Recommendations for Manufacturers and Consumers
For Manufacturers: Stay abreast of regulatory changes, invest in quality assurance, and be transparent with consumers. Proactive engagement with the FDA's processes is essential for success and longevity in the market.
For Consumers: Be vigilant in selecting supplements. Look for evidence of FDA certification, understand the labeling, and choose products from reputable manufacturers.
The FDA's certification is not just a regulatory hurdle but a commitment to excellence. Both manufacturers and consumers play a role in upholding the standards it sets, ensuring the dietary supplement industry remains a trusted source of health and wellness products.
This comprehensive analysis of the FDA's certification process for dietary supplements provides a clear picture of its significance and the responsibilities it entails for both manufacturers and consumers. As the industry continues to grow and evolve, the FDA's role will undoubtedly adapt, but its commitment to public health and safety will remain steadfast.



One Response